What Word Describes the Cooling of Gas by Expansion
Identify and explain some of the everyday applications and consequences of thermal expansion. On this page we will see why expanding gases feel cool.
Which state of matter best describes the sun.

. Avail 25 off on study pack. Condensation liquefaction Which word describes the change of substance from gas to liquid. The ideal gas law describes the behavior of a hypothetical gas in which molecules do not interact with each other except for totally elastic collisions.
What term describes cooling of a gas by expansion. Describe qualitatively the thermal expansion of solids liquids and gases. Thermal expansions of solids liquids and gases.
Adiabatic processes often occur very _. The gas does work on the surrounding atmosphere and so must lose some of its energy since energy is conserved. The adiabatic no heat exchanged expansion of a gas may be carried out in a.
If compression or expansion of gas takes place with no flow of heat energy either into or out of the gas - the process is said to be isentropic or adiabatic. Expansion and Compression of a Gas. Consider the ideal gas law.
Describes the compression or expansion of a gas such that no heat enters or leaves a system. Expansion and Contraction in Solids Liquids and Gases Some materials expand on heating and some contract on cooling. Expansion and Compression of a Gas.
Depending on the upstream-to-downstream pressure ratio the temperature at choke can be much lower than expected. As it sat around the temperature gradually became the same as the surrounding temperature. Storm waves of 2 m amplitude and 8 ms velocity occur on the breakwater of the prototype.
Aphanitic-Fine grain size quick cooling Phaneritic-Coarse grain size- slow cooling Porphyritic-mixture of grain sizes mixture of cooling times Glassy-instant cooling visicular-contains tine holes from gas bubbles in lava-spongelike FragmentalPyro-Pieces of. Vortex tube cooling 3. States that the heat added to a system is equal to the systems increase in internal energy and the external work done by the system.
As the CO 2 gas leaves the nozzle of the cylinder it expands. The gas cooling effect will cause heavy hydrocarbons to drop out. The effect is named after James Prescott Joule and William Thomson 1st Baron Kelvin who discovered it in 1852It followed upon earlier work by Joule on Joule expansion in which a gas undergoes free expansion in a vacuum and the temperature is unchanged if the gas is ideal.
The temperature can easily drop to below ice point resulting in ice-plugging if water exists. V gas volume m 3 ft 3 suffix 1 denotes initial conditions and suffix 2 denotes final conditions. Fast expansion is an adiabatic process and there is no loss of energy to the surrounding.
What describes Uranus and Neptune. Physics 6B - Winter 2011 Homework 4 Solutions. Isentropic or adiabatic CompressionExpansion Processes.
Elastic cooling Special refrigeration method Thermoelectric cooling No moving part at all. Elastic collisions will result in no net energy change. Losing energy will imply cooling down.
What describes the initial of clouds of dust and gas. Part A In an adiabatic process there is no heat transferred to or from the system ie. A very dramatic illustration of a gas cooling as it expands is the formation of dry ice from a carbon dioxide fire extinguisher.
Gas Cooling on Expansion. According to this law if volume increases and pressure is held constant temperature would increase. What term describes cooling of a gas by expansion.
A cold gas thruster is a type of rocket engine which uses the expansion of a pressurized gas to generate thrust. You can find out more about cooling by evaporation on page 11. Starting early can help you score better.
Heating makes the particles that form the material expand or become loose. The statement is a relation between energy of the gas and work being done by the gas. Expansion Driven Heating or Cooling of Gases A Topic Brief About the Joule-Thomson Effect its Industrial Applications When a compressed real gas expands irreversibly and adiabatically across a flow restriction element such as an orifice or valve not only does the pressure of the gas decrease but its temperature can also either spontaneously cool or heat.
When the feed gas is available at high pressure the gas high pressure can be used to generate cooling by isenthalpic expansion or JouleThomson cooling. Voyager since 1980 Semiconductor cooling small. It is simply the law of energy conservation.
Expansion of a gas. R11 cm r22 cm U1400 V U20 V. Cold gas thrusters have been referred to as.
The rst law of thermodynamics then reduces to dQ dW dUdU dW 1 where dW is the amount of work done by the gas and dUis the change in internal energy. As opposed to traditional rocket engines a cold gas thruster does not house any combustion and therefore has lower thrust and efficiency compared to conventional monopropellant and bipropellant rocket engines. It would be better to say that gases lose energy when they provide work during expansion.
However even if the hydrocarbon. Cooling makes the particles that form the material contract or become tight. Gases expand the.
Then on expansion the gas cools down. This low temperature is due to the JouleThomson cooling effect that is a sudden gas expansion below the nozzle causes a significant temperature drop. The mathematical equation for an ideal gas undergoing a reversible ie no entropy generation adiabatic process can be represented by the polytropic process equation where P is pressure V is volume and for this case n γ where C P being the specific heat for constant pressure C V being the specific heat for constant volume γ is the adiabatic index and f is the number of.
Liquid to gas at the internal cooling site expansion and gas to liquid transition at the compressor site. Find and sketch the potential between two coaxial cylinders of radii r1 and r2 having potential U1 and U2 respectively. A model of a harbor is made on the length ratio of 3601.
Describe qualitatively the effect of a change of temperature on the volume of a gas at constant pressure. Just for the sake of exactitude the change in temperature on a isenthalpic-adiabatic drop in pressure a free expansion is called the Joule-Thomson coefficient. Removal of the heavy hydrocarbons can then meet the hydrocarbon dew point specification.
What word describes a gas changing to a liquid. There are tow reasons for that cooling on expansion. Word describes a gas changing to a liquid.
The amount of expansion differs in solids liquids and gases. 25362 Chemical 17 Nov 03 0654. The gas heated up when it was compressed.
Utilizing Peltier effect Opposite of Thermoelectric generator eg.

12 2 First Law Of Thermodynamics Thermal Energy And Work Texas Gateway
2 1 Internal Energy Chemistry Libretexts

6 7 Expansion And Contraction Of Materials Particle Model Of Matter Siyavula
Why Does Gas Heat Up When Contracted And Cool When Expanded Quora

Thermal Expansion Physics Britannica
In A Joule Expansion Why Does The Temperature Of An Ideal Gas Remains Constant But The Temperature Of A Real Gas Decreases Quora

Joule Thomson Effect Definition Derivation Formula And Examples

What Is The First Law Of Thermodynamics Article Khan Academy

Thermal Contraction Expansion What Is Thermal Contraction Video Lesson Transcript Study Com

Thermal Contraction Expansion What Is Thermal Contraction Video Lesson Transcript Study Com
The First Law Of Thermodynamics

12 2 First Law Of Thermodynamics Thermal Energy And Work Texas Gateway

Http Www Aplustopper Com Expansion Contraction In Solids Liquids Gases Solid Liquid Gas The Expanse Contractions
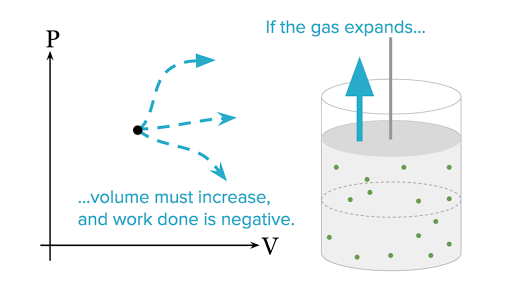
What Are Pv Diagrams Article Khan Academy

What Is The First Law Of Thermodynamics Article Khan Academy

Q17 Describe An Experiment To Lido

Solar Dhw Diagram Solar Water Heater Water Heater Installation Solar Water



Comments
Post a Comment